
DE
|
EN
> Linker und Spacer
PC Linker BMN (Photocleavable Linker) (Internal modification)
Photocleavable Linker – light-induced cleavage of labelled oligonucleotides
Photolabile linker structures are generally understood to mean a connection between two molecules through a short linker which can be cleaved by irradiation with light of a certain wavelength (UV light at 300-400 nm). The supply of energy in the form of light represents a simple, well-regulated "switch function", since it enables targeted, time-coordinated cleavage of the biomolecules.

Figure 1: Structure of the internal photocleavable linker BMN (PC linker BMN) bound to an oligo.
Even the short exposure to high-energy light using a Maldi measurement is sufficient to display the decomposition products of an oligo with an internal PC linker.
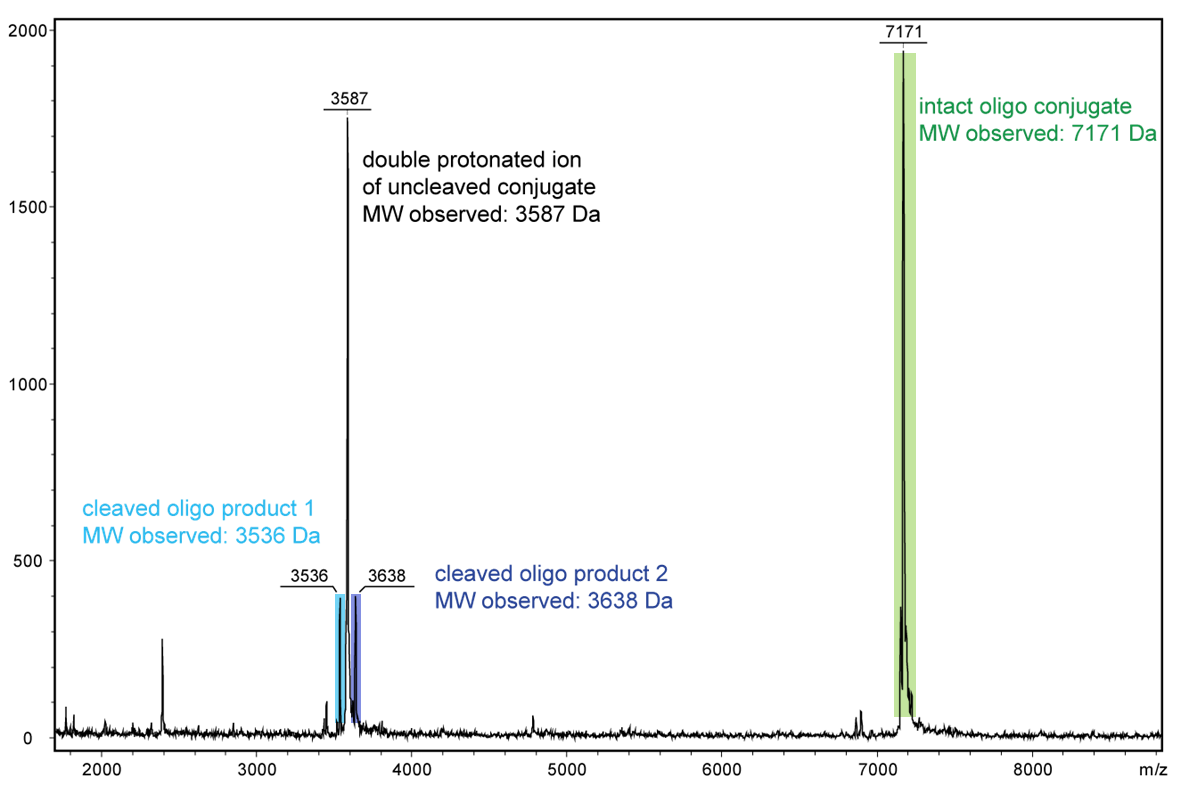
Figure 2 shows the result of the Maldi analysis of a dT20 oligonucleotide with an internal photolabile linker (PC). The complete oligonucleotide with a molecular weight of 7171 Da is shown in the large peak on the right side (highlighted in green): 5´-antraquinone-TTT TTT TTT T-PC-TTT TTT TTT T-BMN Q535-3´(MW 7171 Da ).
The almost equally large decay products due to the PC linker placed in the middle of the sequence are highlighted in blue:
Product 1 5´-Phos-TTT TTT TTT T-BMN Q535-3´(highlighted in light blue, MW 3536 Da) and product 2 5´-antraquinone-TTT TTT TTT T-PC modified-3´ (highlighted in dark blue, MW 3638 Da).
The decomposition products shown here were generated by a short laser pulse, the majority of the oligo remains uncleaved (green peak). The large left peak shows the double-protonated ion of the uncleaved conjugate (MW 3587 Da).
a)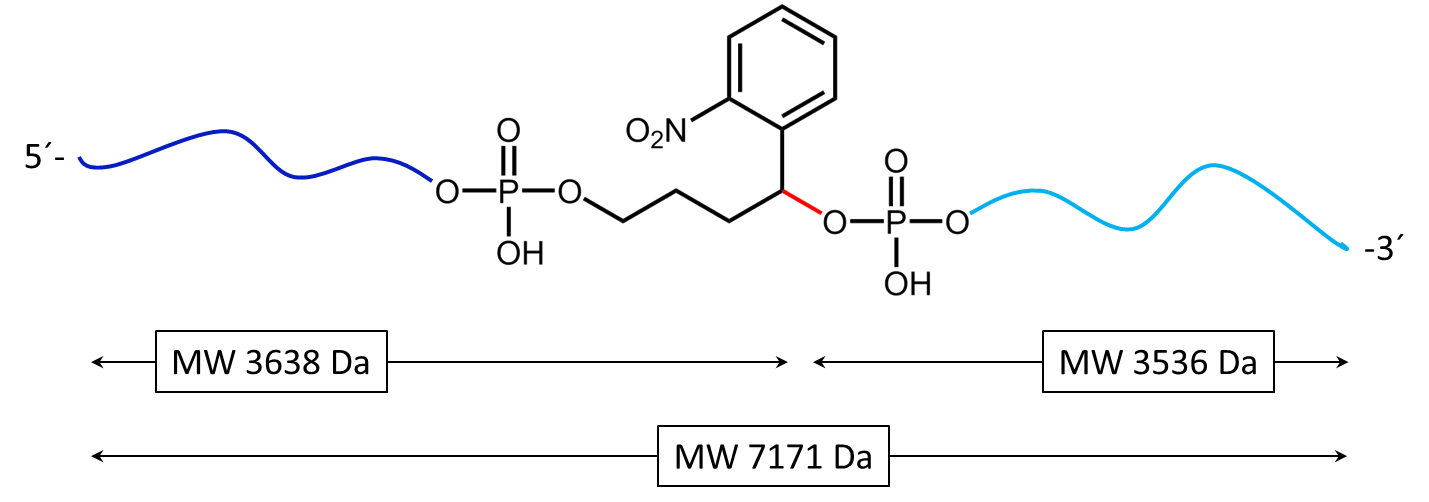
b)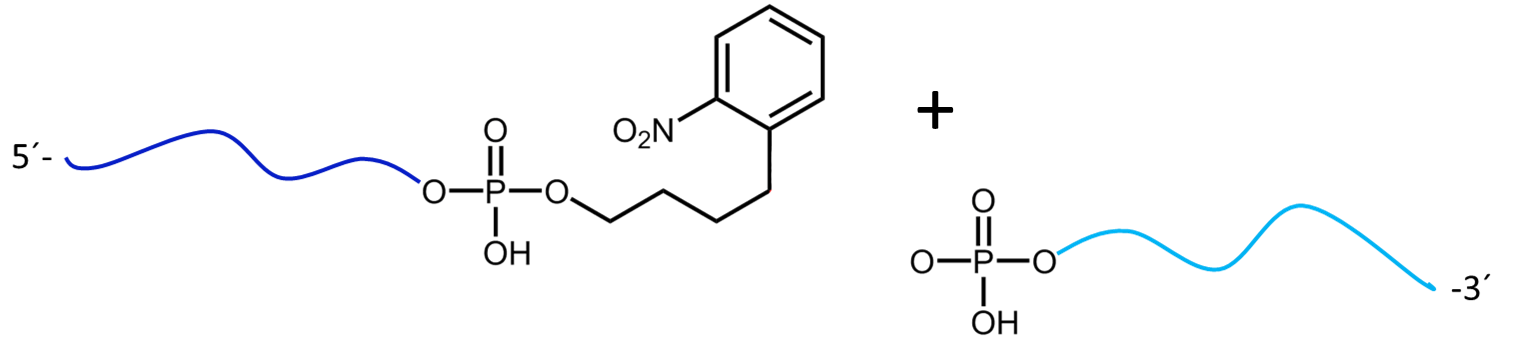
Figure 3a) shows the cleavage reaction of the oligo conjugate with Maldi laser irradiation. The cleavage site is shown in red. 3b) shows the resulting cleavage products after the partial decomposition of the PC linker.
For a complete cleavage of the photolabile linker in the oligo, light exposure takes longer, usually a few minutes. If the exposure is sufficiently long, the linker itself also disintegrates. In the following Figure 4, another cleavage product is shown after 30 minutes of exposure. Here, the linker which has already been cleaved completely falls off the oligo.
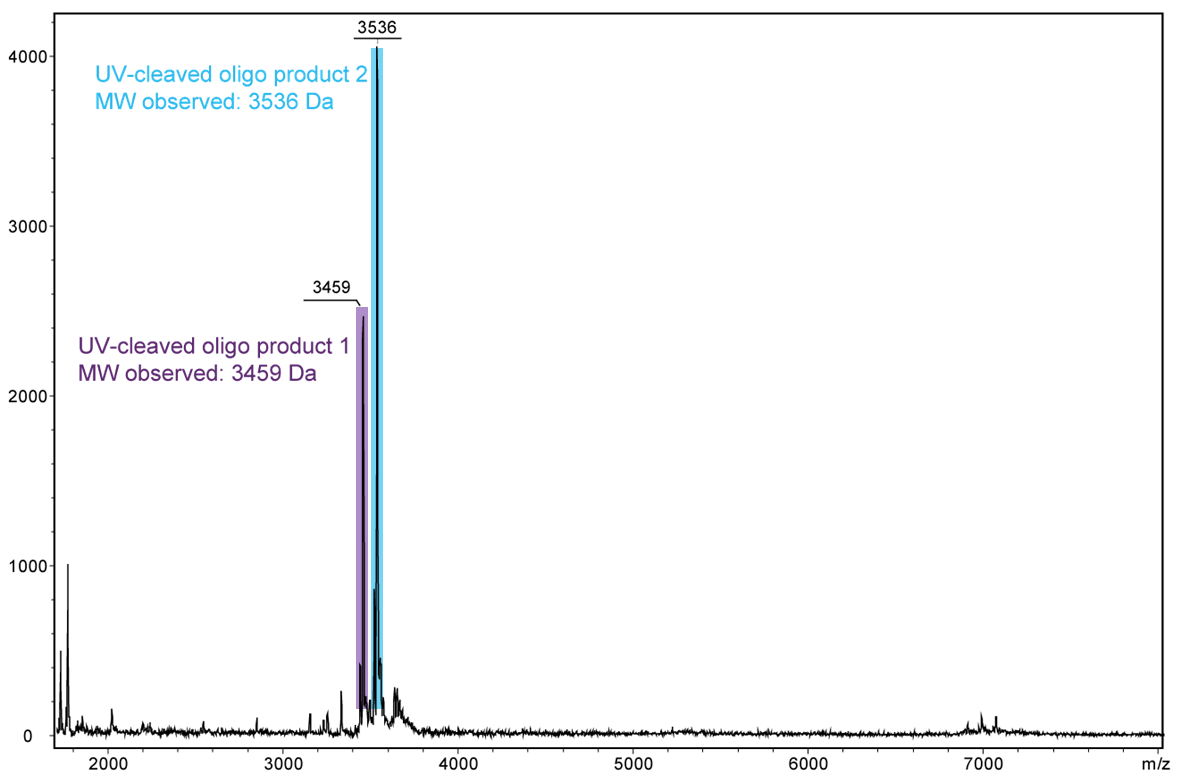
Figure 4) shows the result of the Maldi analysis of the oligo conjugate in aqueous solution after irradiation with UV light at 365 nm for 30 min. If the PC linker is irradiated with UV light, it disintegrates and completely falls off the oligo. The spectrum shows the two cleavage products of the conjugate:
5´-antraquinone-TTT TTT TTT T-Phos-3´ (highlighted in purple, 3459 Da) and 5´-Phos-TTT TTT TTT T-BMN Q535-3´ (highlighted in light blue, 3536 Da).
The oligo conjugate is completely cleaved after UV exposure.
a)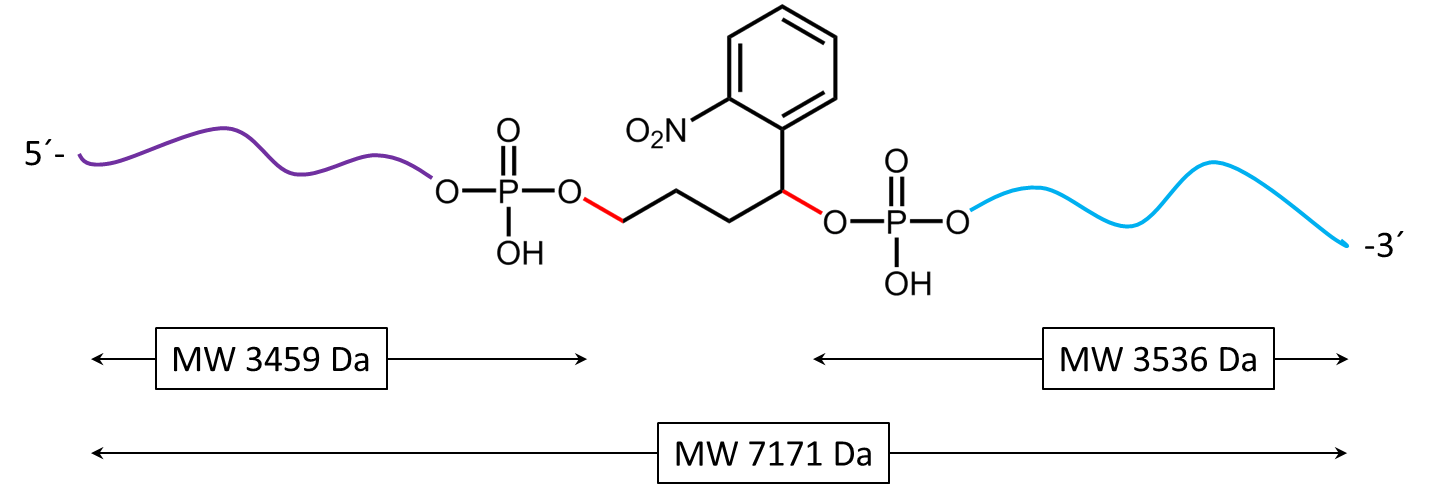
b)
Figure 5a) shows the cleavage reaction of the oligo conjugate when exposed to UV radiation. The cleavage sites are shown in red. 5b) shows the resulting cleavage products after the PC linker has completely disintegrated.
Photolabile linker structures are generally understood to mean a connection between two molecules through a short linker which can be cleaved by irradiation with light of a certain wavelength (UV light at 300-400 nm). The supply of energy in the form of light represents a simple, well-regulated "switch function", since it enables targeted, time-coordinated cleavage of the biomolecules.

Figure 1: Structure of the internal photocleavable linker BMN (PC linker BMN) bound to an oligo.
Even the short exposure to high-energy light using a Maldi measurement is sufficient to display the decomposition products of an oligo with an internal PC linker.

Figure 2 shows the result of the Maldi analysis of a dT20 oligonucleotide with an internal photolabile linker (PC). The complete oligonucleotide with a molecular weight of 7171 Da is shown in the large peak on the right side (highlighted in green): 5´-antraquinone-TTT TTT TTT T-PC-TTT TTT TTT T-BMN Q535-3´(MW 7171 Da ).
The almost equally large decay products due to the PC linker placed in the middle of the sequence are highlighted in blue:
Product 1 5´-Phos-TTT TTT TTT T-BMN Q535-3´(highlighted in light blue, MW 3536 Da) and product 2 5´-antraquinone-TTT TTT TTT T-PC modified-3´ (highlighted in dark blue, MW 3638 Da).
The decomposition products shown here were generated by a short laser pulse, the majority of the oligo remains uncleaved (green peak). The large left peak shows the double-protonated ion of the uncleaved conjugate (MW 3587 Da).
a)

b)

Figure 3a) shows the cleavage reaction of the oligo conjugate with Maldi laser irradiation. The cleavage site is shown in red. 3b) shows the resulting cleavage products after the partial decomposition of the PC linker.
For a complete cleavage of the photolabile linker in the oligo, light exposure takes longer, usually a few minutes. If the exposure is sufficiently long, the linker itself also disintegrates. In the following Figure 4, another cleavage product is shown after 30 minutes of exposure. Here, the linker which has already been cleaved completely falls off the oligo.

Figure 4) shows the result of the Maldi analysis of the oligo conjugate in aqueous solution after irradiation with UV light at 365 nm for 30 min. If the PC linker is irradiated with UV light, it disintegrates and completely falls off the oligo. The spectrum shows the two cleavage products of the conjugate:
5´-antraquinone-TTT TTT TTT T-Phos-3´ (highlighted in purple, 3459 Da) and 5´-Phos-TTT TTT TTT T-BMN Q535-3´ (highlighted in light blue, 3536 Da).
The oligo conjugate is completely cleaved after UV exposure.
a)

b)

Figure 5a) shows the cleavage reaction of the oligo conjugate when exposed to UV radiation. The cleavage sites are shown in red. 5b) shows the resulting cleavage products after the PC linker has completely disintegrated.

