
Lipophilic modifications
Due to the ribose-phosphate backbone oligonucleotides are hydrophilic and dissolve well in water or buffer. Adding a lipophilic modification to the oligonucleotide shifts its solubility, making it amphiphilic, so that it may be directed to lipophilic target sites such as membranes, lipids or hydrophobic proteins. The lipophilic moiety also enhances passage through lipid membranes and uptake into cells or tissues.
The utility of lipophilic modifications on oligonucleotides has been widely explored for drug delivery, in antisense, siRNA, and aptamer research, and for immunostimulatory oligos. They also found use in nanobiotechnology.
| Modification | 5´ | 3´ |
|---|---|---|
| Cholesterol | x | x |
| α-Tocopherol | x | |
| Trolox | x | x |
| Palmitate | x | |
| Stearate | x | |
| Distearoyl-Lipid | x | |
| Ibuprofen | x | x |
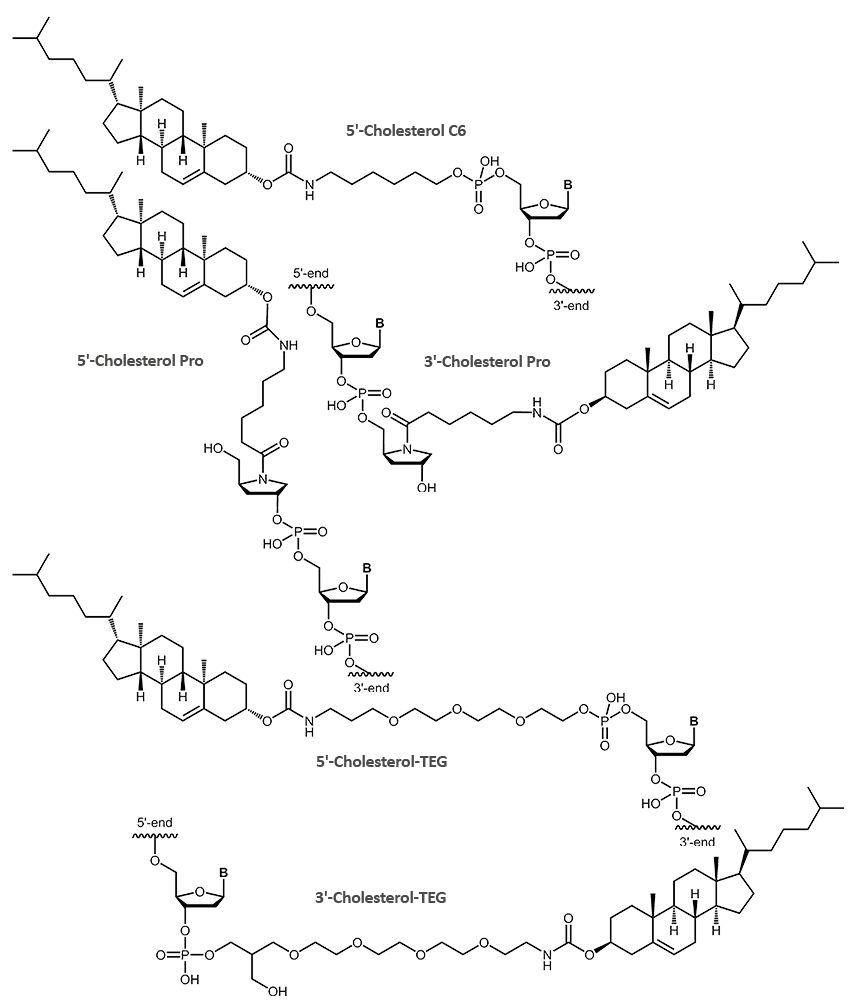
Cholesterol
Cholesterol
Enhanced delivery and uptake of DNA antisense oligonucleotides or siRNA has been demonstrated for cholesterol-modified oligonucleotides. The highly lipophilic character of the steroid cholesterol leads to a higher uptake into cells and thereby an increased activity.
The hydrophobicity of the cholesterol can be exploited to attach oligonucleotide-conjugates to membranes or vesicles. A feature that also has been used to build artificial DNA-nanostructures associated with lipid membranes.
 |
Literature:
1. Modification of antisense phosphodiester oligodeoxynucleotides by a 5' cholesteryl moiety increases cellular association and improves efficacy. Krieg AM, Tonkinson J, Matson S, Zhao Q, Saxon M, Zhang LM, Bhanja U, Yakubov L, Stein CA; Proc. Natl. Acad. Sci USA (1993), 90, 1048-1052.
2. Silencing of microRNAs in vivo with ‘antagomirs‘. Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M; Nature (2005), 438, 685 - 689.
3. Synthetic lipid membrane channels formed by designed DNA nanostructures. Langecker M, Arnaut V, Martin TG, List J, Renner S, Mayer M, Dietz H, Simmel FC; Science (2012), 338. 932 – 936.
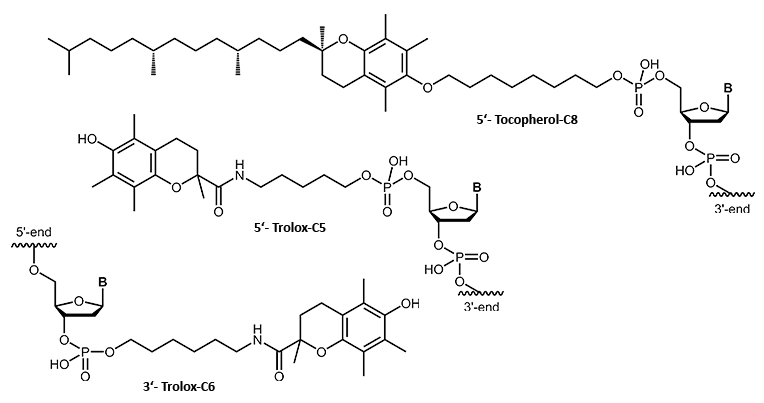
Tocopherol, Trolox
Tocopherol, Trolox
α-Tocopherol is a naturally occurring vitamin (vitamin E group). Conjugated to an oligonucleotide it adds a lipophilic character. As tocopherol can be recognised and transported by body specific proteins and carriers, the tocopherol modification may also serve as a means to route delivery of the conjugated oligonucleotide within the organism.
The tocopherol derivative Trolox also belongs to the vitamin E group. In comparison to tocopherol, Trolox is more hydrophilic and further shows strong antioxidant properties. Due to the improved intercellular effect and cellular uptake, new opportunities for oligo conjugates with these lipophilic modifications arise in the field of antisense oligonucleotides.
Furthermore, in combination with a fluorescent dye, Trolox is able to "stabilise" the dye and protect it against fading.
 |
Literature:
1. Efficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherol. Nishina K, Unno T, Uno Y, Kubodera T, Kanouchi T, Mizusawa H, Yokota T; Mol Ther. (2008), 16:734–740.
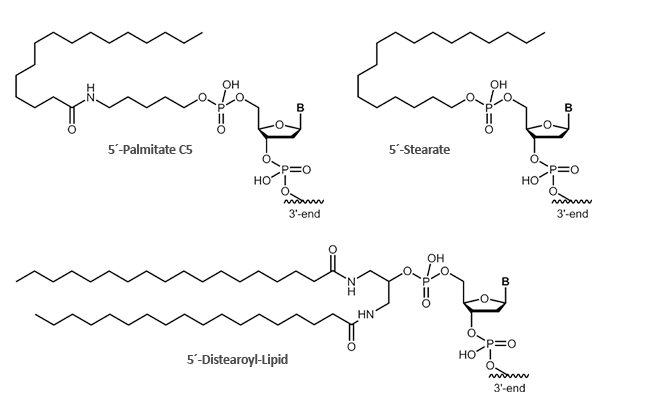
Stearate, Palmitate, Distearoyl-Lipid
Stearate, Palmitate, Distearoyl-Lipid
Fatty acids bound to oligonuclotides render the conjugate amphiphilic so that the conjugates can be attached to lipid phases, bilayers or vesicle. The oligo-moiety is thus presented to the aqueous phase, while the fatty acid dips into the lipid phase. An increased uptake through cell membranes is also observed.
biomers.net offers different fatty acids:
- Palmitate
- Stearate
- Distearoyl-Lipid
 |
Literature:
1. DNA modification of live cell surface. Borisenko GG, Zaitseva MA, Chuvilin AN, Pozmogova GE; Nucleic Acids Res. (2009), 37, e28.
2. Lipid modification of GRN163, an N3' -> P5' thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Herbert BS, Gellert GC, Hochreiter A, Pongracz K, Wright WE, Zielinska D, Chin AC, Harley CB, Shay JW, Gryaznov SM; Oncogene (2005), 24, 5262–5268.
3. General method for modification of liposomes for encoded assembly on supported bilayers. Yohina-Ishii, Miller GP, Kraft ML, Kool ET, Boxer SG; J. Am. Chem. Soc. (2005), 9, 1356- 1357.
Ibuprofen
Antisense oligonucleotides and aptamers with ibuprofen
A new approach to significantly increase lifetime of aptamers in cell is the coupling of small molecules such as ibuprofen to DNA oligonucleotides.
Enzymatic degradation by endo- and exonucleases, as well as liver and kidney clearance of comparatively small molecules like aptamers and antisense oligonucleotides (25-30 kDa) from circulation can limit lifetime of these possible drugs and thus even reduce their therapeutic potential.
Drugs like ibuprofen are able to bind albumin or other serum proteins, as well as ibuprofen conjugates (e.g. chemically modified oligonucleotides with ibuprofen). 3
Due to increased molecular weights of these complexes (antisense oligonucleotide / aptamer-ibuprofen-albumin) the clearance by liver and kidney is hindered and resistence against enzymatic nucleases (endo- and exonucleases) is also increased. 1,2
In this way, lifetime as well as intracellular distribution and thus the pharmacological availability of the modified antisense oligonucleotides or aptamers can be improved.2
At the target site, the oligonucleotide-ibuprofen-conjugate can disassociate from albumin carrier in order to get fully effective.2
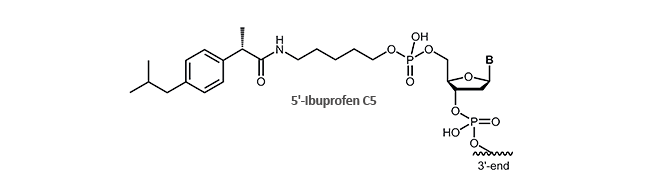
Now biomers.net offers ibuprofen as 5´-and /or 3´-modification for DNA oligonucleotides.
Literature:
1. Predicting the Uncertain Future of Aptamer-Based Diagnostics and Therapeutics. Bruno, JG; Molecules (2015), 20, 6866-6887; doi:10.3390/molecules20046866.
2. A Review of Therapeutic Aptamer Conjugates with Emphasis on New Approaches. Bruno, JG; Pharmaceuticals (2013), 6, 340-357; doi:10.3390/ph6030340.
3. Improving Antisense Oligonucleotide Binding to Human Serum Albumin: Dramatic Effect of Ibuprofen Conjugation. Manoharan M, Inamati GB, Lesnik EA, Sioufi NB, Freier SM; Chembiochem. (2002); 3(12):1257-60.

