
Oligonucleotides for electrochemical detection
Oligos with redox modifications
Methylene Blue, Ferrocene, Anthraquinone, Viologen, Atto MB2 and Nile Blue –
oligonucleotides with redox-active reporter molecules
In recent years, a variety of new approaches of oligonucleotide-based electrochemical biosensors, so-called
E-sensors, have been developed. This form of biosensors is an extremely sensitive and selective method for detecting certain DNA sequences (e.g. SNP detection). All DNA-based electrochemical sensors are based on the interaction between a target molecule in solution, a target-binding probe, and a solid electrode surface.
Many of the oligonucleotides are labelled at one end with a redox modification, such as Methylene Blue, Ferrocene, Anthraquinone, Viologen, Atto MB2 or Nile Blue and linked via the other, free terminus to a conductive surface (e.g. electrode).
The covalent attachment of the redox-active oligo probes to conductive surfaces such as gold electrodes is achieved via a terminal thiol or thioctic acid modification.
For effective detection, several redox-active reporters are available:
In the absence of a complementary strand, redox-active oligo probes take a specific conformation with a defined distance between probe and electrode.
The hybridisation of a complementary target molecule to the surface-bound oligo leads to a change in conformation. This influences the electron transfer between the redox unit and the electrode, whereby potential change can be detected by suitable measuring methods.
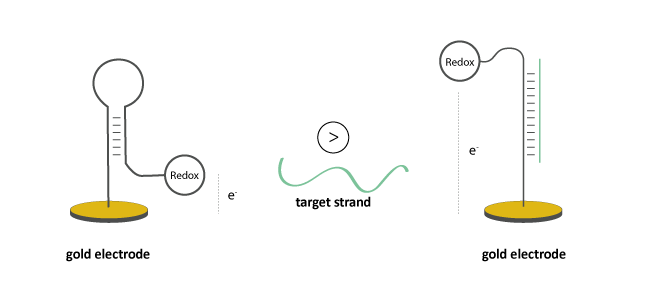
Figure 1: Conformational change of an oligo probe with redox modification (Methylene Blue, Ferrocene, Anthraquinone, Viologen, Atto MB2 or Nile Blue) after hybridisation of a complementary target strand. The change in electrode potential can be finally detected.
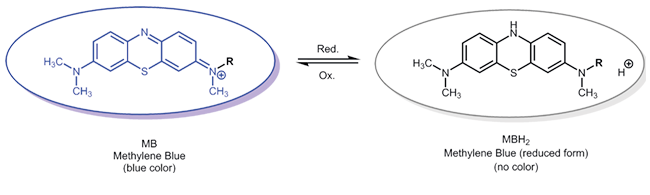
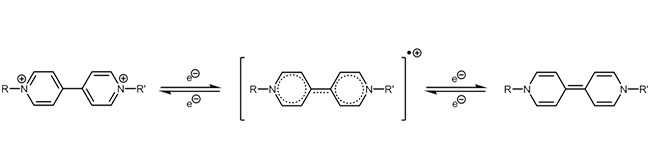
Oxidation-reduction reaction of redox-active reporter molecules - Methylene blue
Ferrocene
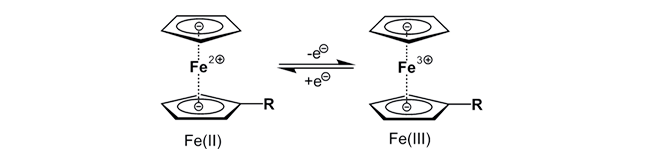
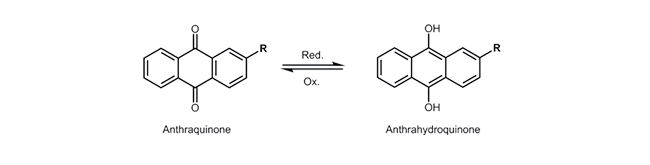
Anthraquinone
Viologen
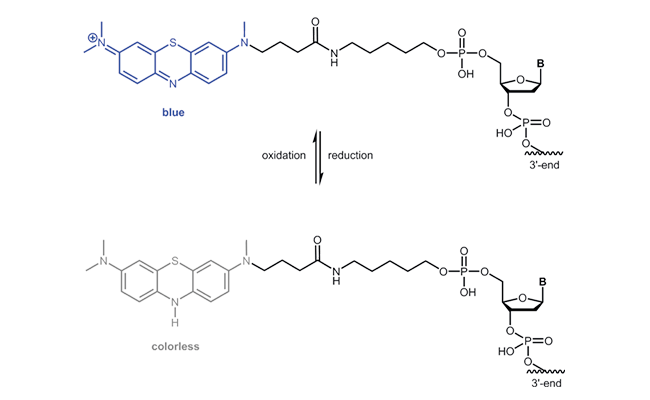
Atto MB2
Nile Blue
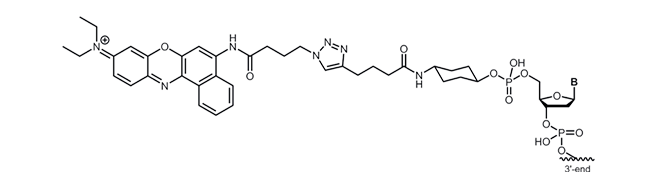
biomers.net offers the redox modifications Methylene Blue, Ferrocene, Anthraquinone, Viologen, Atto MB2 or Nile Blue as 3´-and 5´-modification.
In addition to the redox label at the 5´-end, the oligonucleotide can be labelled on request at the 3´-end with a thiol or thioctic acid.
For further details please contact us at any time.
Tel +49 731 70 396 0 ǀ info@biomers.net
Literature:
1. Comparing the Properties of Electrochemical-Based DNA Sensors Employing Different Redox Tags. Kang D, Zuo X, Yang R, Xia F, Plaxc, KW, White R; Anal Chem. (2009), 81(21): 9109–9113.
2. Ferrocene-oligonucleotide conjugates for electrochemical probing of DNA. Ihara T, Maruo Y, Takenaka S, Takagi M; Nucleic Acids Res (1996), 24. 4273.
3. DNA biomolecular-electric encoder and decoder devices constructed by multiplex biosensors. Kang D, White RJ, Xia F, Zuo X, Vallée-Bélisle A, Plaxco KW; NPG Asia Materials (2012), 4, doi:10.1038/am.2012.1.
4. Uridine-Conjugated Ferrocene DNA Oligonucleotides: Unexpected Cyclization Reaction of the Uridine Base. Yu CJ, Yowanto H, Wan Y, Meade TJ, Chong Y, Strong M, Donilon LH, Kayyem JF, Gozin, M, Blackburn GFJ; Am. Chem. Soc. (2000), 122, 6767-6768.
5. 2´-Ribose-Ferrocene Oligonucleotides for Electronic Detection of Nucleic Acids. Yu CJ, Wang H, Wan Y, Yowanto H, Kim JC, Donilon LH, Tao C, Strong M, Chong YJ; Org. Chem. (2001), 66, 2937-2942.
Oligos for surface immobilisation
Modifications for the immobilisation on conductive surfaces
Various strategies are available for the covalent attachment of oligonucleotides to conducting surfaces. In addition to coupling to gold surfaces via thiol and thioctic acid compounds it is also possible to bind oligonucleotides via
4-Carboxymethylaniline to conductive surfaces such as graphite.
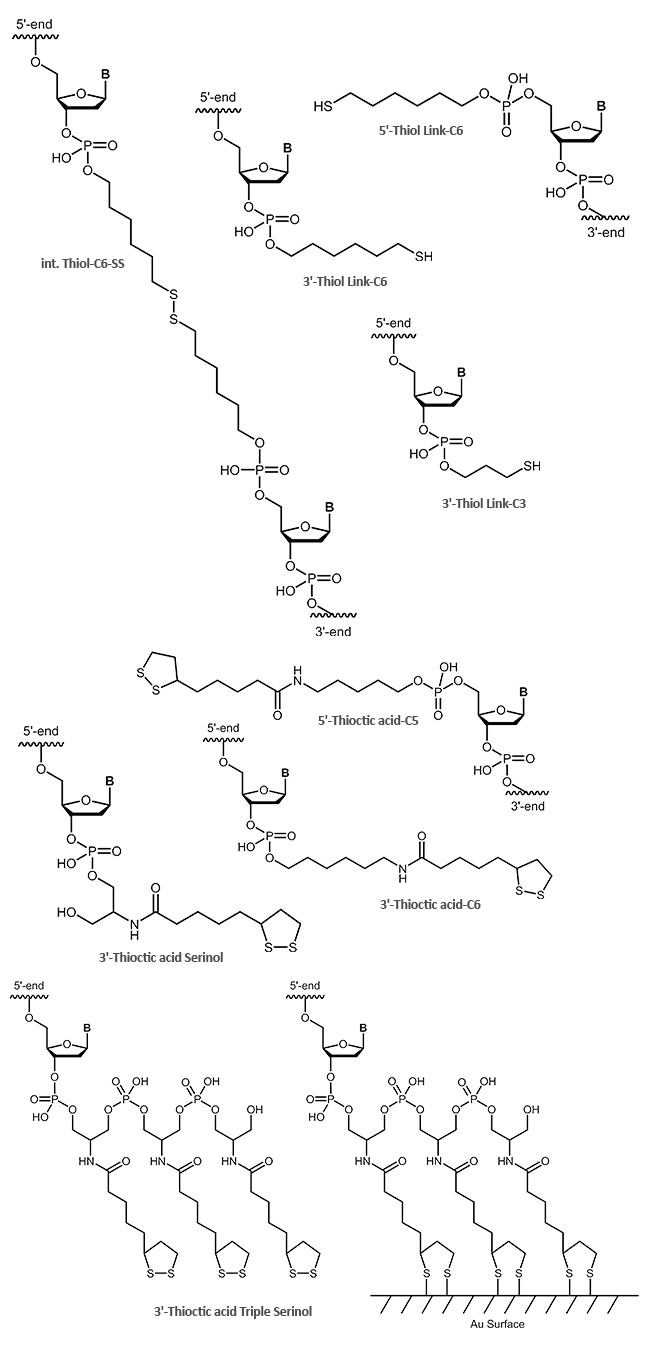
Thiol and thioctic acid modification on oligos for attachment to gold surfaces
In addition to the simple terminal reactive thiol (SH group), the thioctic acid modification is also suitable for the coupling of oligonucleotides to gold surfaces. Due to its two sulfur atoms, thioctic acid can bind even more strongly to gold electrodes. Even higher binding affinities can be achieved with the thioctic acid triple, which binds tightly to the gold surface with six sulfur atoms.
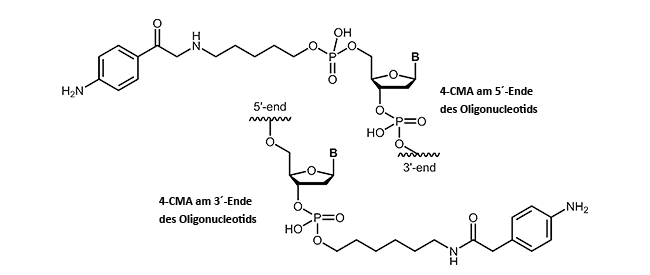
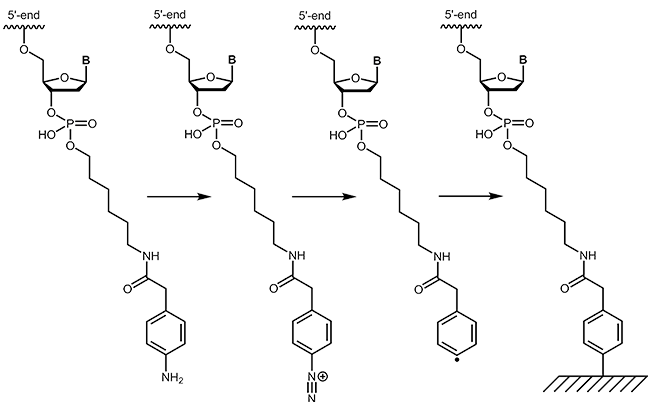
4-Carboxymethylaniline-modified oligonucleotides
4-Carboxymethylaniline (4-CMA) allows to immobilise coupled biomolecules to appropriate prepared surfaces. In this way, the binding of oligonucleotides to conductive surfaces (e.g. graphite) enables the detection of currents. By coupling, either to the 5´-or 3´-end of oligonucleotides, the molecules are covalently bonded in the desired orientation to the surface.
Literature:
- Diazonium-Protein Adducts for Graphite Electrode Microarrays Modification: Direct and Addressed Electrochemical Immobilization. Corgier BP, Marquette CA, Blum LJ; Journal of the American Chemical Society, (2005), 127, 18328-18332.
- A versatile method for direct and covalent immobilisation of DNA and proteins on biochips. Corgier BP, Laurent A, Perriat P, Blum LJ, Marquette CA; Angewandte Chemie International (2007), 46, 4108-4110.
- On-Chip Chemiluminescent Signal Enhancement using Nanostructured Gold-Modified Carbon Microarrays. Corgier BP, Li F, Blum LJ, Marquette CA; Langmuir (2007), 23(16), 8619-8623.

