Duplex Stability
A variety of molecular biological methods are based on the specific hybridization of the complementary DNA strands. Hybridization due to Watson-Crick base pairing also defines the specificity of the binding. In addition to the H-bonds formed in base pairing, affinity and stability of the duplex are dependent on stacking effects between the neighboring base pairs (hydrophobic and electrostatic interactions).
Duplex stability therefore is dependent on length and GC content of a sequence. For shorter oligonucleotides duplex stability and melting temperature Tm may decrease in as much that affinity and binding strength are not sufficient to apply stringent conditions during hybridization and to ensure specific binding. With the introduction of modified bases duplex stability can be enhanced to allow an improved, specific hybridization of the oligonucleotide to its target.
| Modification | Abbreviation | ΔTm | Substitutes |
|---|---|---|---|
| Locked nucleic acid | LNA | +2 to +9.6° C | |
| Minor Groove Binder | MGB | +10 to +20° C | |
| C-5 Propynyl-deoxycytidine | pdC | +2,8° C | dC |
| C-5 Propynyl-deoxyuridine | pdU | +1,7° C | dT |
| Aminoethyl-phenoxazine-deoxycytidine | AP-dC (G-clamp) | +7 to +21° C | dC |
| 5-Methyl-deoxycytidine | 5-Me-dC | +1,3° C | dC |
| 2-Amino-deoxyadenosine | 2-Amino-dA | +3° C | dA |
| Trimethoxystilbene | Trimethoxystilbene | +10° C | |
| Pyrene | Pyrene | +10° C |
The change in melting temperature ΔTm given in the table is a reference number. It is influenced by the sequence context and therefore may vary.
Minor Groove Binder
Minor Groove Binder (MGB)
MGB-modified oligonucleotides show increased melting temperature (Tm) and specificity and can form very stable duplexes with complementary DNA sequences. Here, the base composition of the oligos strongly influences the melting temperature of the modified oligos.
When binding the probe to the target sequence, minor groove binders (MGB) bind to the minor groove of the DNA. The crescent shape of the MGB CDPI3 (Dihydropyrroloindolecarboxylate Tripeptide CDPI3) allows an exact spatial adaptation to the curved structure of the minor groove, thus allowing an intercalation between the sugar-phosphate chains of the DNA backbone. This isohelical conformation is stabilised mainly by van der Waals forces and hydrophobic interactions.
According to this, an extremely high stability of the double-stranded molecule can be achieved, so that even very short DNA probes can hybridise very efficiently to the target DNA.
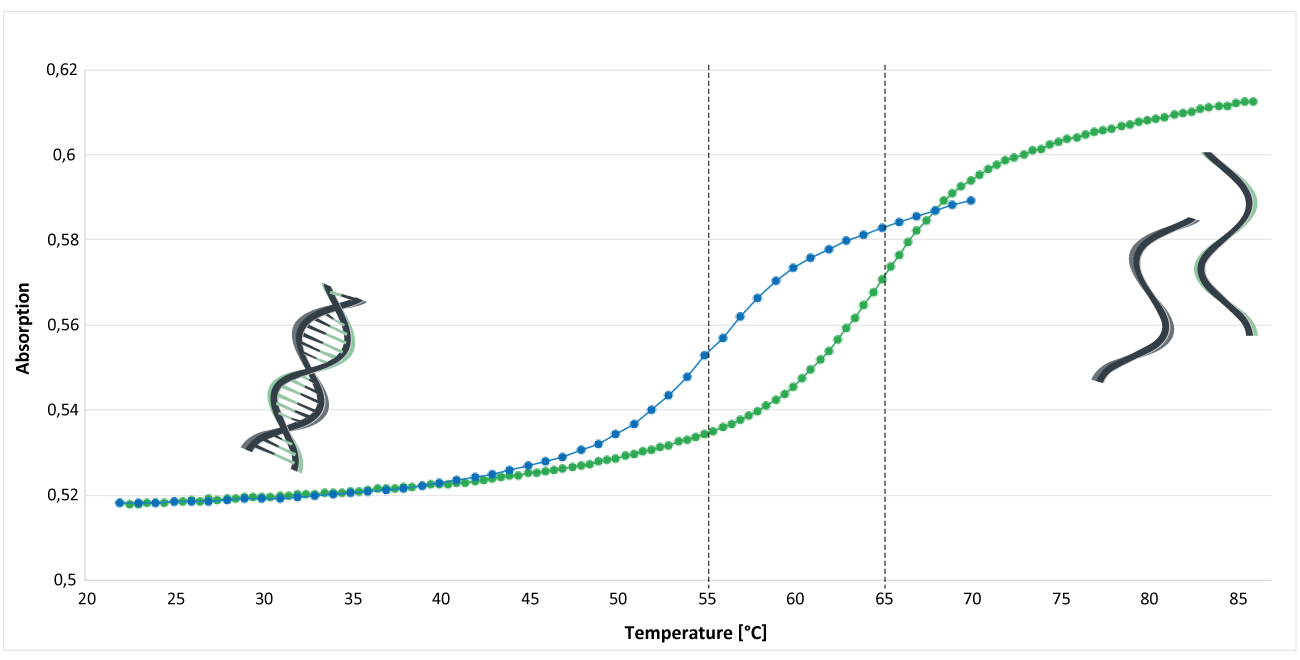
Figure 1 shows the analysis of the melting curve of an MGB CDPI3-modified and an unmodified oligo. The analysis was performed in phosphate buffer (pH 7.4). The concentration of Na+ was 30 mM; the concentration of the DNA single strands was approx. 1.3 μM. The inflection point of the melting curve indicates the melting temperature Tm of the oligos.
The blue curve represents an unmodified EUB338 oligo: 5'-gctgcctcccgtaggagt-3 '. The melting temperature of this oligo is around 55°C.
The green curve shows the melting curve of an MGB CDPI3-modified oligo. The EUB oligo was labelled at the 3 'end with a MGB CDPI3 modification: 5'-gctgcctcccgtaggagt-MGB CDPI3. Here, the melting temperature is around 65°C.
Under these conditions, comparing these two oligos, an increase in melting temperature of about 10°C can be detected.
A detailed ordering information you can find here.
For any further information or help, please contact the biomers.net customer service.
Literature:
1. Minor Groove Binder-Conjugated DNA Probes for Quantitative DNA Detection by Hybridization-Triggered Fluorescence. Afonina IA, ReedMW, Lusby E, Shishkina IG, Belousov YS; BioTechniques (2002) 32:940-949 .
2. 3′-Minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures. Kutyavin IV, Afonina IA, Mills A, Gorn VV, Lukhtanov EA, Belousov ES, Singer MJ, Walburger DK, Lokhov SG, Gall AA, Dempcy R, Reed MW, Meyer RB, Hedgpeth J; Nucleic Acids Research (2000), Volume 28, Issue 2, Pages 655–661.
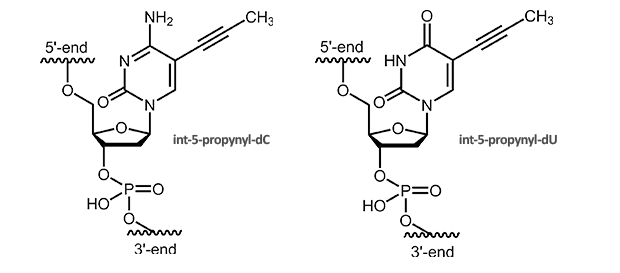
C-5 Propynyl-Pyrimidines
C-5 Propynyl-deoxycytidine and C-5 Propynyl-deoxyuridine
C5-Propynyl derivatives of the pyrimidine bases stabilize the duplex due to improved stacking and additional hydrophobic interactions. Substituting dC with C5-propynyl-dC increases the Tm of around 2.8° C per modification, substituting dT with C5-propynyl-dU of around 1.7° C per modification.
 |
Literature:
1. Propynyl groups in duplex DNA: stability of base pairs incorporating 7-substituted 8-aza-7-deazapurines or 5-substituted pyrimidines. He J, Seela F; Nucleic Acids Res. (2002), 5485-5496.
2. Oligodeoxynucleotides containing C-5 propyne anlogs of 2‘-deoxyuridine and 2‘-deoxycytidine. Froehler BC, Wadwani S, Terhorst TJ, Gerrard SR; Tetrahedron Lett. (1992), 33, 5307-5310.
3. Design of modified oligodeoxyribonucleotide probes to detect telomere repeat sequences in FISH assays. Hacia JG, Novotny EA, Mayer RA, Woski SA, Ashlock MA, Collins FS, Nucleic Acids Res. (1999), 27, 4034-4039.
4. Evaluation of C-5 propynyl pyrimidine-containing oligonucleotides in vitro and in vivo. Shen et al. Antisense Nucleic Acid Drug Dev. (2003), 13, 129-142.
C5-propynyl-pyrimidine nucleosides are covered by a patent. Corresponding phosphoramidite building blocks are offered by Glen Research (www.glenresearch.com) and a license agreement allows biomers.net to produce C5-propynyl-pyrimidine modified oligonucleotides for research use only.*
* This product is covered by patents or patents pending owned by Isis Pharmaceuticals, Inc. (“Isis”). Purchase of this product includes a limited license to use this product solely for internal research. This license specifically excludes (and you have no right to use this product for): (a) therapeutic or diagnostic applications (including products or services that incorporate this product), (b) any in vivo toxicity/safety study in support of an investigational new drug application (or foreign counterpart), (c) resale (including sale of any products or services that incorporate this product) or (d) gene functionalization activities (including products or services that incorporate data derived from gene functionalization activities) if such activities have commercial applications, any and all of which require a separate license from Isis. Neither this product nor any product created through its use may be used in human clinical trials. In the event you have a separate agreement with Isis regarding this product which explicitly states that the foregoing is not applicable to you, your use of this product will be governed by the terms of such agreement. In no event does the limited license with the purchase of this product expand or alter the scope of the license granted pursuant to such agreement.
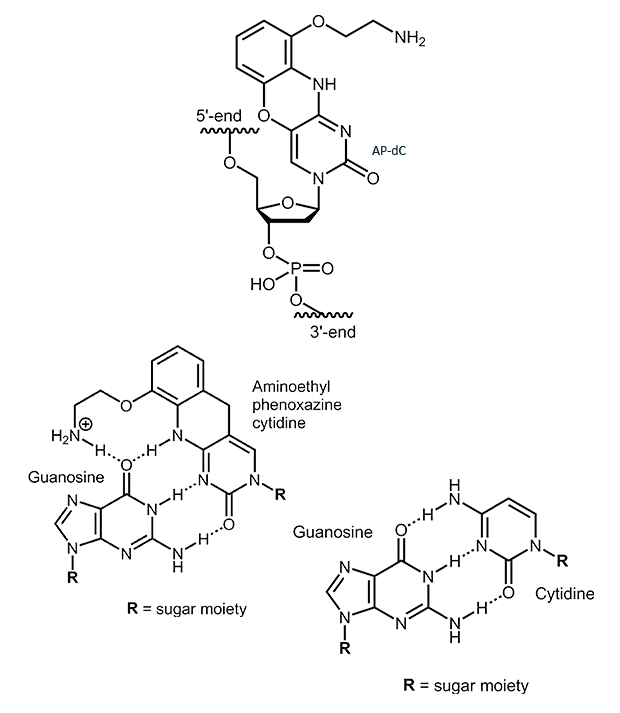
AP-dC
2-Aminoethyl-phenoxazine-deoxycytidine (AP-dC, G-Clamp)
2-Aminoethyl-phenoxazine-deoxycytidine (AP-dC) is an analogue of cytidine. The additional ring system allows the formation of a fourth H-bond when base pairing with guanosine. This can lead to an increase in melting temperature of 7° to 21° C. The magnitude of the increase, however, is largely influenced by the neighboring base pairs and therefore strongly sequence dependent.
 |
Literature:
1. A cytosine analogue capable of clamp-like binding to a guanine in helical nucleic acids. Lin KY, Matteucci MD; J. Am. Chem. Soc. (1998), 120, 8531-8532.
2. A cytosine analog that confers enhanced potency to antisense oligonucleotides. Flanagan WM, Wolf JJ, Olson P, Grant D, Lin K, Wagner RW, Matteucci MD; Proc. Natl. Acad. Sci. (1999), 96, 3513-3518.
3. Binding affinities of oligonucleotides and PNAs containing phenoxazine and G-clamp cytosine analogues are unusually sequence-dependent. Ortega JA, Blas JR, Orozco M, Grandas A, Pedroso E, Robles J; Org. Lett. (2007), 9, 4503-4506.
AP-dC (G-Clamp) is covered by a patent. A phosphoramidite building block of AP-dC is offered by Glen Research (www.glenresearch.com) and a license agreement allows biomers.net to produce AP-dC modified oligonucleotides for research use.*
* This product is covered by patents or patents pending owned by Isis Pharmaceuticals, Inc. (“Isis”). Purchase of this product includes a limited license to use this product solely for internal research. This license specifically excludes (and you have no right to use this product for): (a) therapeutic or diagnostic applications (including products or services that incorporate this product), (b) any in vivo toxicity/safety study in support of an investigational new drug application (or foreign counterpart), (c) resale (including sale of any products or services that incorporate this product) or (d) gene functionalization activities (including products or services that incorporate data derived from gene functionalization activities) if such activities have commercial applications, any and all of which require a separate license from Isis. Neither this product nor any product created through its use may be used in human clinical trials. In the event you have a separate agreement with Isis regarding this product which explicitly states that the foregoing is not applicable to you, your use of this product will be governed by the terms of such agreement. In no event does the limited license with the purchase of this product expand or alter the scope of the license granted pursuant to such agreement.
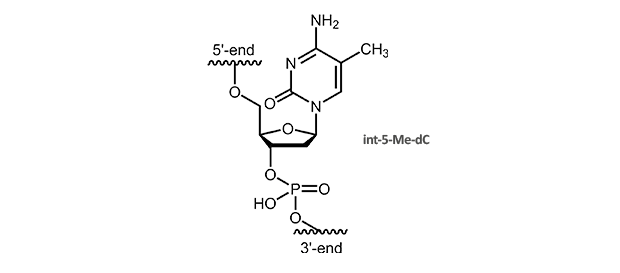
5-Methyl-dC
5-Methyl-deoxycytidine
5-Me-pyridines enhance duplex stability due to improved stacking interactions. A substitution of dC by 5-Me-dC also raises the melting temperature of triplex helices. The Tm is increased around 1.3° C per modification, multiple substitutions are additive, also for a larger number.
 |
Literature:
1. Origins of the large differences in stability of DNA and RNA helixes: C-5 methyl and 2‘-hydroxyl effects. Wang S, Kool ET; Biochemistry (1995), 34, 4125-4132.
2. Poly(pyrimidine)*poly(purine) synthetic DNAs containing 5-methylcytosine form stable triplexes at neutral pH. Lee SL, Woodsworth ML, Latimer LJP, Morgan AR; Nucleic Acids Res. (1984), 16, 8603-8614.
2-Amino-deoxyadenosine
2-Amino-deoxyadenosine (2,6-diaminopurine)
The adenosine analogue 2-amino-deoxyadenosine forms a specific base pair with thymidine. The additional amino group in the 2 position of the adenosine allows the formation of an additional H-bridge, raising melting temperature around 3° C.
 |
Literature:
1. Thermodynamic studies of base pairing involving 2,6-diaminopurine. Cheong C, Tinoco I, Chollet A; Nucleic Acids Res. (1988), 16, 5115-5122.
2. DNA containing the base analogue 2-aminoadenine: preparation, use as hybridization probes and cleavage by restriction endonucleases. Collet A, Kawashima E; Nucleic Acids Res. (1988), 16, 305-317.
3. Oligonucleotides containing 2-aminoadenine and 5-methylcytosine are more effective as primers for PCR amplification than their non-modified counterparts. Lebedev Y, Akopyants N, Azhikina T, Shevchenko Y, Potapov V, Stecenko D, Berg D, Sverdlov E; Genetic Anal. (1996), 13, 15-21.
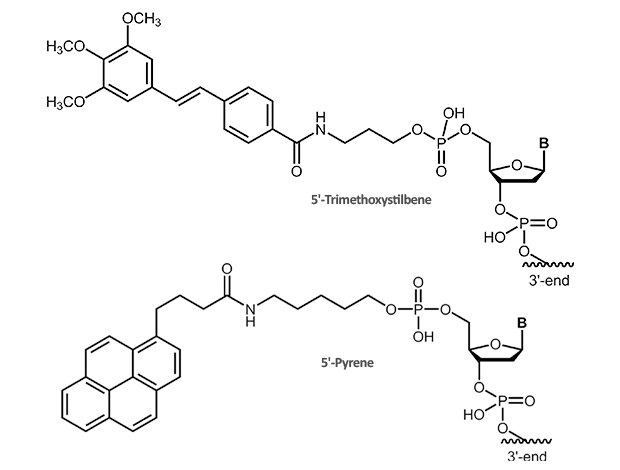
Stilbene, Pyrene Cap
Trimethoxystilbene and Pyrene
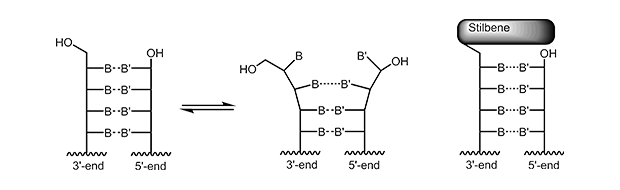
The 5‘-trimethoxystilbene and 5‘-pyrene cap stacks over the terminal base pair. For a short duplex this leads to an increase in melting temperature of approx. +10° C. As the overlay is only possible when the previous bases are in correct Watson-Crick pairing, the 5’-cap also improves discrimination of mismatched bases at the 5’-terminus.
|
|
Literature:
1. 5’-Tethered stilbene derivatives as fidelity- and affinity-enhancing modulators of DNA duplex stability. Dogan Z, Paulini R, Rojas Stütz JA, Narayanan S, Richert C; J. Am. Chem. Soc. (2004), 126, 4762-4763.
2. Clamping down on weak terminal base pairs: oligonucleotides with molecular caps as fidelity-enhancing elements at the 5’- and 3’-terminal residues. Narayanan S, Gall J, Richert C; Nucleic Acids Res. (2004), 32, 2901-2911.